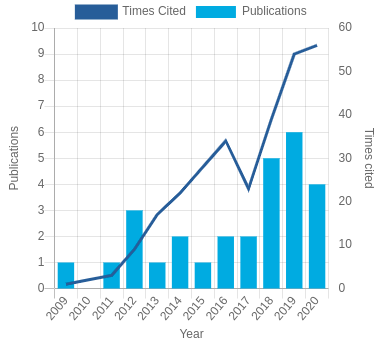
Citation:
Abstract:
We employed PBE and BLYP semi-local functionals and the van der Waals density functional of Dion et al. (2004) (vdW-DF) to investigate structural properties of liquid acetonitrile and methanol. Among those functionals the vdW-DF is the only one that correctly predicts energy minima in inter-molecular interactions between acetonitrile molecules. We found that van der Waals interactions have a negligible effect on H-bonds in methanol chains. However, it significantly increases chain packing resulting in a more dense liquid in comparison to the other two functionals. The overall trend is that the vdW-DF tends to overestimate density and bulk modulus, meanwhile the semi-local functionals tend to underestimate density. Thus, van der Waals interactions play an important role in the properties of liquids in which much stronger dipole-dipole interactions are present.